Cytochrome f project
Cytochrome f project
Together with the Malkin group at U.C. Berkeley we are investigating the structure of site-directed
mutants of cytochrome f in Chlamydomonas. Cytochrome f is a component of the photosynthetic b6f
complex analogous to cytochrome c1 of the mitochondrial bc1 complex. We have excellent crystals of
Wollman's engineered soluble cyt. f
, and have solved the structure by molecular replacement using a
preliminary 2.2Å dataset. The next step is to crystallize constructs which include the
functional mutations that have been characterized by the Malkin lab.
[Cytochrome f sequences]
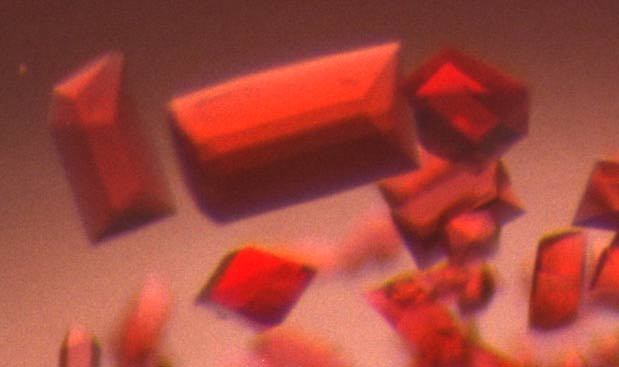
Orthorhombic Crystals of Chlamydomonas cyt f soluble fragment.
The asymmetric unit contains a trimer.
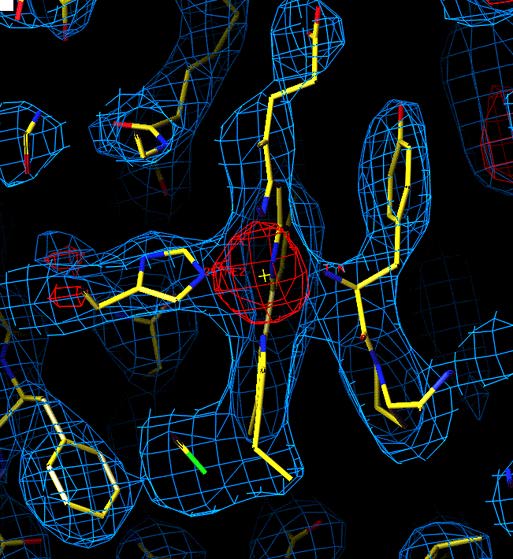
Unrefined structure of Chlammydomonas reinhardti ctyochrome f
in the heme region. (purification and crystallization by Lishar Huang,
data colection by
ZhaoLei Zhang, molecular replacement by Young-in Chi using 1ctm.pdb as
a model)
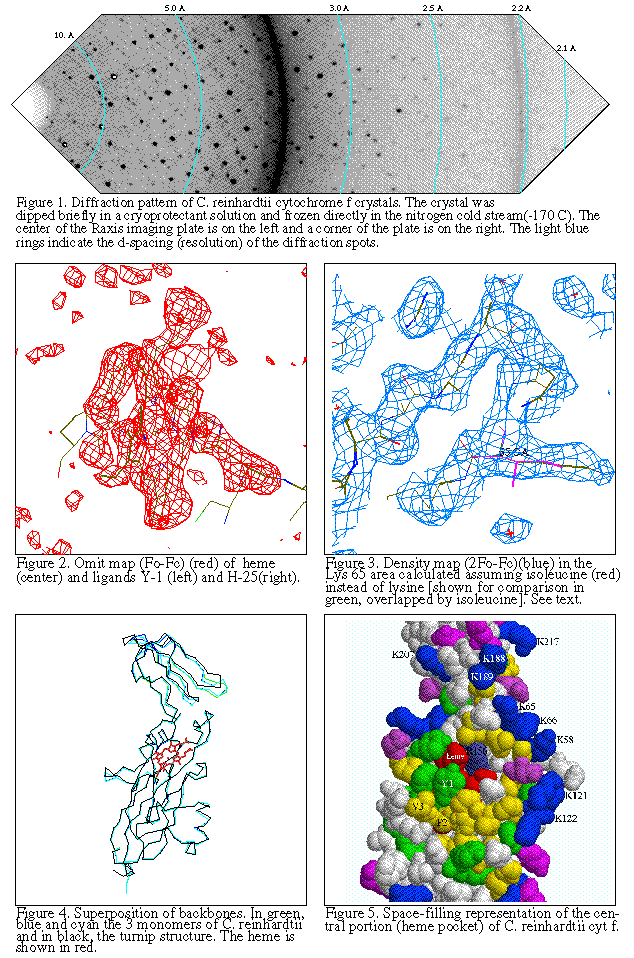
The structure of the "wild-type" truncated Chlamydomonas cytochrome f was finally
published in 1999. You can see some figures
from the paper. Later Xiao-Song Gong of the Malkin Lab combined the functionally
important mutants with the C-terminal truncation of Kuras/Wolman, and Rosie Uenge
expressed huge quantities of the mutant proteins for Li-shar to purify and crystallize.
Data was collected, mailnly at SSRL and the ALS this time, and structures have
been solved and refined for Y1S, P2V, K187E+K188L, and K188L+K189T+K65E mutants.
The mutants crystallized in different space groups than the wild type, P21 for the
N-terminal mutants and P6122 for the lysine patch mutants, but no unexpected
structural changes were observed.
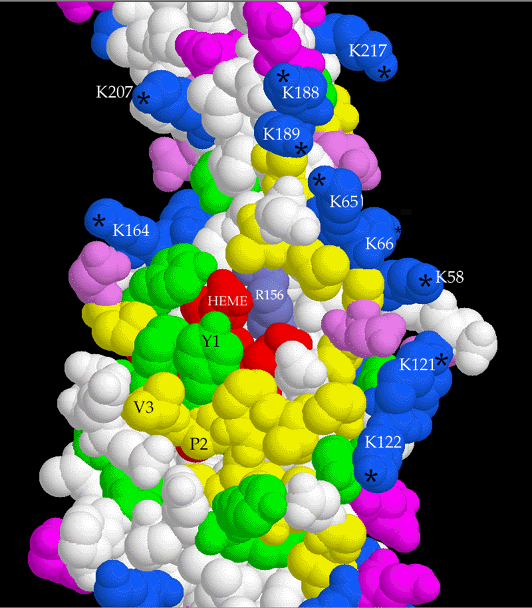
One unrelated observation was that in some crystals the first heme-lgating
cystein residue has two conformations, i.e. the thioether bond is partially broken.
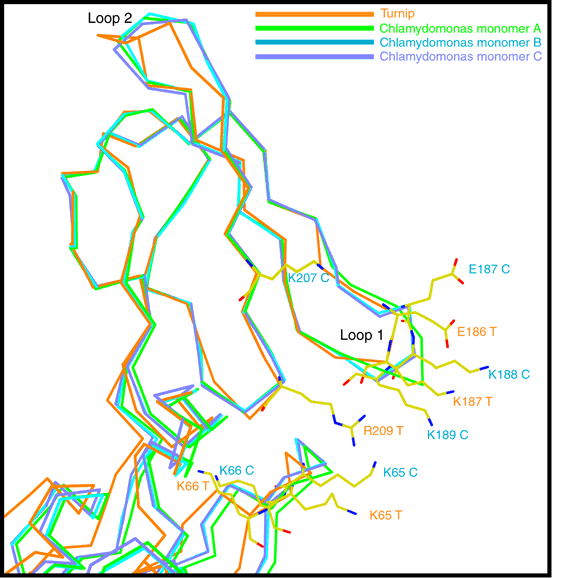
Dimeric relation between cyt f monomers in crystals
Another observation was that a particular interprotein contact tends to occur in the different
crystal forms, in which two monomers are related by a proper 2-fold or slightly distorted
proper two-fold. As far as we know the purified protein is a monomer, still this indication
of self-affinity is suggestive of a physiological role in the dimeric b6f complex. Such a role
is made even less likely by the orientation of hemes in the putative dimer, which is
inconsistent with that measured by epr or linear dichroism spectroscopy.
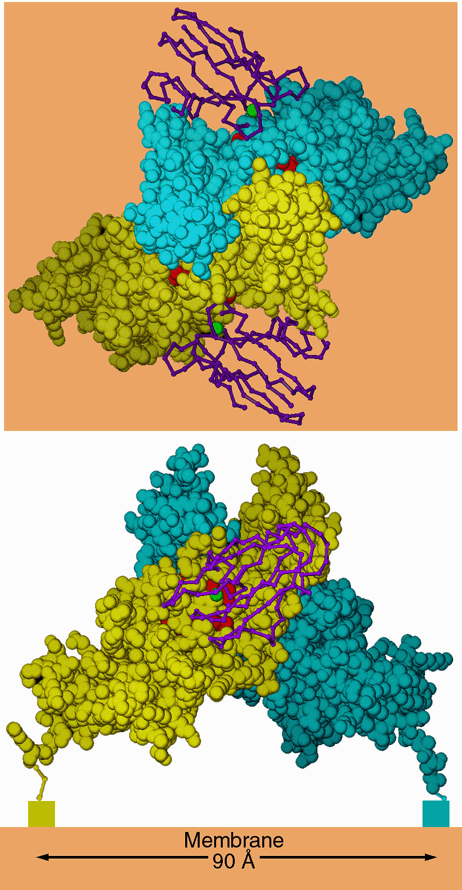
Figure 7. Dimer-like noncrystallographic symmetry observed in the crystal of C. reinhardtii cytochrome f. Above is a "top" view, looking down the 2-fold axis of symmetry. Below is a side view, looking parallel to a hypothetical membrane plane constructed perpendicular to the 2-fold (orange). The blue and yellow space-filling models are monomers A and B from the crystal of C. reinhardtii cytochrome f. The hemes are shown as red space-filling models. The rectangles below the C termini in the lower panel represent the lumenal ends of the transmembrane helices. The magenta backbone drawings are plastocyanin from the NMR structure of the plastocyanin-cytochrome f complex (2PCF), oriented with the operators that best superimpose cytochrome f of that structure on each monomer of the C. reinhardtii dimer. The copper atom of plastocyanin is shown as a green sphere. The interactions betwen these two monomers in the crystal may be similar to those involved in stabilizing a physiological dimer in vivo (see text). In this figure monomer B is taken directly from the structure 1CFM; monomer A is transformed by crystallographic symmetry operator [1/2+x, 1/2-y, 1-z] because the chosen asymmetric unit does not include this dimer.






